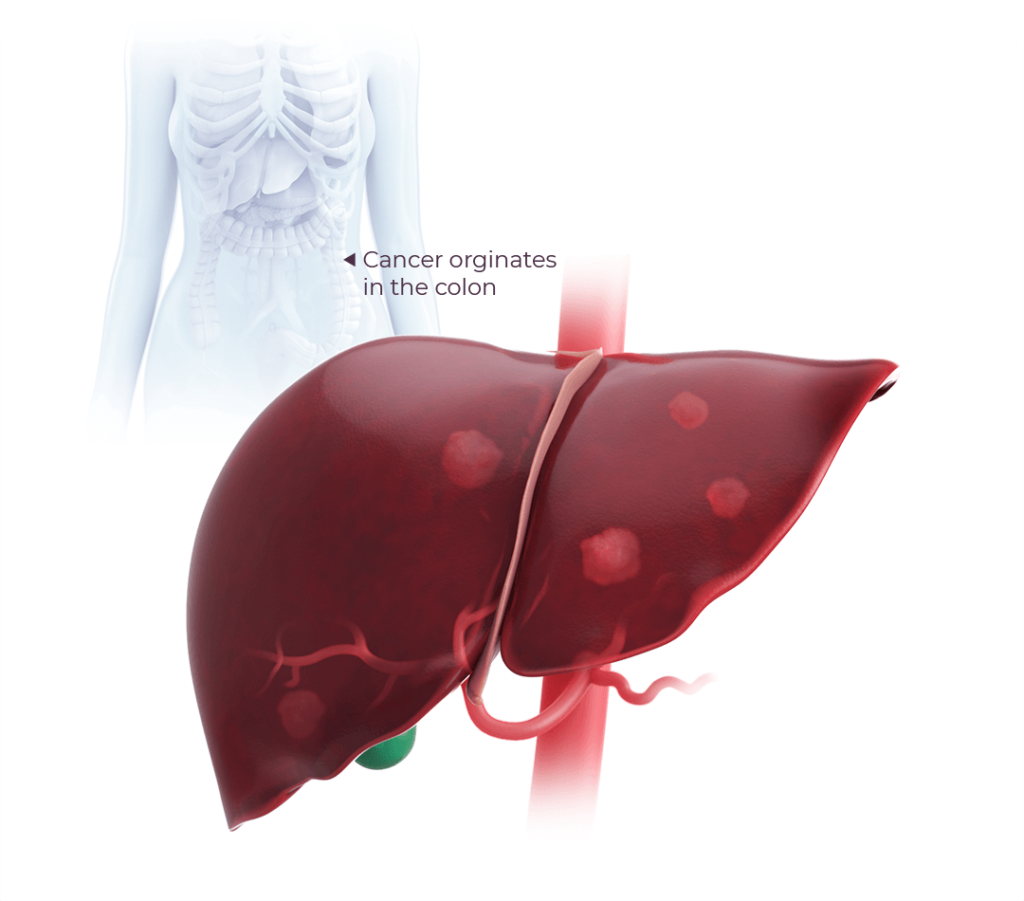
Colorectal cancer (CRC) is the fourth most common major cancer in the United States, as approximately 1 in 25 people will be diagnosed with CRC during their lifetime.1
Overall, 5-year survival rates have increased over time, largely due to increased screening procedures. Despite this, in 20% of cases CRC has already metastasized at the time of diagnosis2 which represents a serious and life-threatening disease with a poor prognosis. Nearly 70% of CRC metastases occur in the liver with the median survival for patients of about 9 months.2
TriSalus’ CRC with liver metastases clinical program will initially evaluate our investigational TLR9 agonist, nelitolimod (also known as SD-101), delivered deep into the vasculature of the liver tumors using our proprietary, FDA cleared device. Traditionally, TLR9 agonists like nelitolimod are not administered intravenously but by direct injection into superficial tumors, making treatment of liver metastases very difficult.
TriSalus is studying the delivery of nelitolimod directly into the arteries supplying the liver in order to distribute the drug to CRC liver metastases within the organ, irrespective of size, number and location of tumors. Infusion by the TriSalus device for the Pressure-Enabled Drug Delivery™(PEDD™) method improves targeted delivery of therapy into high-pressure tumors using a standard intraarterial procedure.
Because immune cells are suppressed throughout the liver and micro-metastases or undetectable additional tumors may be present, tissue that appears normal is also treated with nelitolimod. Importantly, the nelitolimod treatment is being given in combination with a systemic (or intravenous) immunotherapy to enable treatment of tumors within the liver as well as tumors that may be present in other parts of the body.
Nelitolimod is in clinical development and has not been approved in the US or globally.
We are studying the combination of nelitolimod with PEDD to enable immunotherapies in the liver and pancreas. Our clinical research is focused on bringing this potentially transformative treatment platform to patients.
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.