


Myeloid-Derived Suppressor cells (MDSCs)
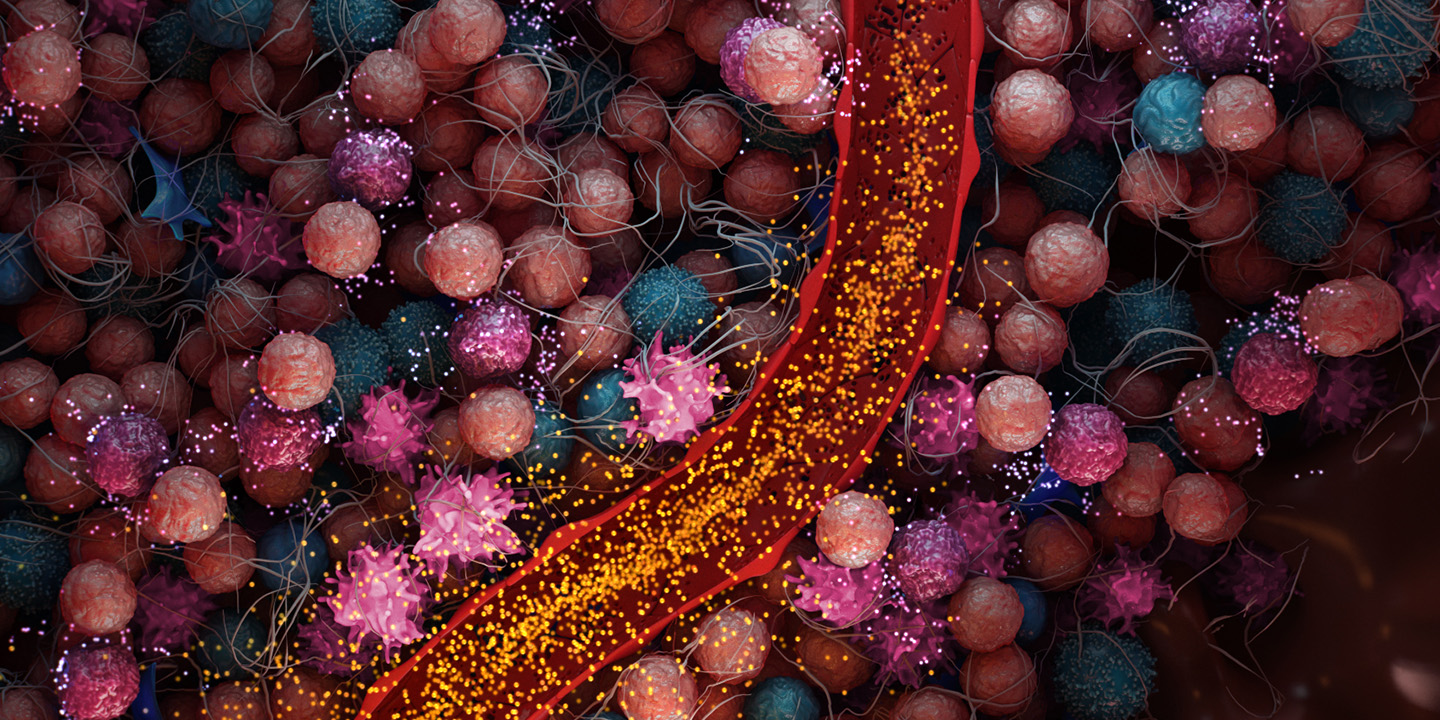
Immunosuppressive MDSCs are recruited to the TME. TLR9 agonists have been shown to reprogram and eliminate MDSCs, reducing their immunosuppressive effects.6

Natural Killer (NK) Cells
In tumors, NK cells are suppressed through various pathways but have shown reactivation upon TLR9 stimulation.6

Plasmacytoid Dendritic Cells (pDCs)
pDCs are suppressed in the TME but have shown reactivation through TLR9.6





Immunosuprresive MDSCs are recruited to the tumor microentvironment. TLR9 agnoists have been shown to reprogram and eliminate MDSCs, reducing their immunosupressive effects. [Ref]

In tumors, NK cells are suppressed through various pathways but have shown reactivation upon TLR9 stimulation.[Ref]

Immunosuprresive MDSCs are recruited to the tumor microentvironment. TLR9 agnoists have been shown to reprogram and eliminate MDSCs, reducing their immunosupressive effects. [Ref]

pDCs are suppressed in the tumor microenvironment but have shown reactivation through TLR9. [Ref]
Tumors within the liver and pancreas have specific immunosuppressive pathways that render therapies less effective.7 MDSCs accumulate within the liver and pancreas in the presence of cancer.6,8
The immunosuppressive environment of these organs and the high level of MDSCs is believed to enable a tumor to evade the immune system9 and induce further resistance to immunotherapy approaches such as checkpoint inhibitors and CAR-T therapeutics.10,11
High levels of MDSCs have been observed in a number of cancers including primary liver, liver metastases, pancreatic cancer and melanoma.12,13
Stimulating TLR9 may promote anti-tumor activity by activating the immune system to help attack solid tumors and convert suppressive immune cells into more immunostimulatory subtypes.1,2
TLR9 agonists have been shown to convert immunosuppressive MDSCs into immunostimulatory macrophages, while also affecting other cell types.14,15 TriSalus is currently studying whether MDSC reprogramming can be used to enable immunotherapeutic treatments, including checkpoint inhibitors and cell therapy.
Myeloid cell depletion has been shown to sensitize tumors to systemic therapies, including chemotherapy, radiation, checkpoint blockade and chemoimmunotherapy.13,16
Our investigational candidate nelitolimod is a TLR9 agonist aimed at reactivating the immune system.
We are currently conducting clinical studies to determine if our investigational TLR agonist, nelitolimod (also known as SD-101), has the potential to enhance the benefit of other immuno-oncology agents when infused by the Pressure-Enabled Drug Delivery™ (PEDD™) method.
Trisalus is studying the ability of nelitolimod to reprogram and deplete MDSCs and enable immunotherapeutic treatments for liver tumors, liver metastases and pancreatic tumors.
Our proprietary technology has been shown to overcome intratumoral pressure and reopen blood vessels.
TriSalus’ platform addresses two significant treatment barriers directly in the liver and pancreas. We believe that the administration of immune-enabling therapies (i.e., investigative nelitolimod) by the Pressure-Enabled Drug Delivery™ (PEDD™) method provides a means to treat these hard-to-reach tumors.
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.