Nelitolimod is in clinical development and has not been approved in the US or globally.
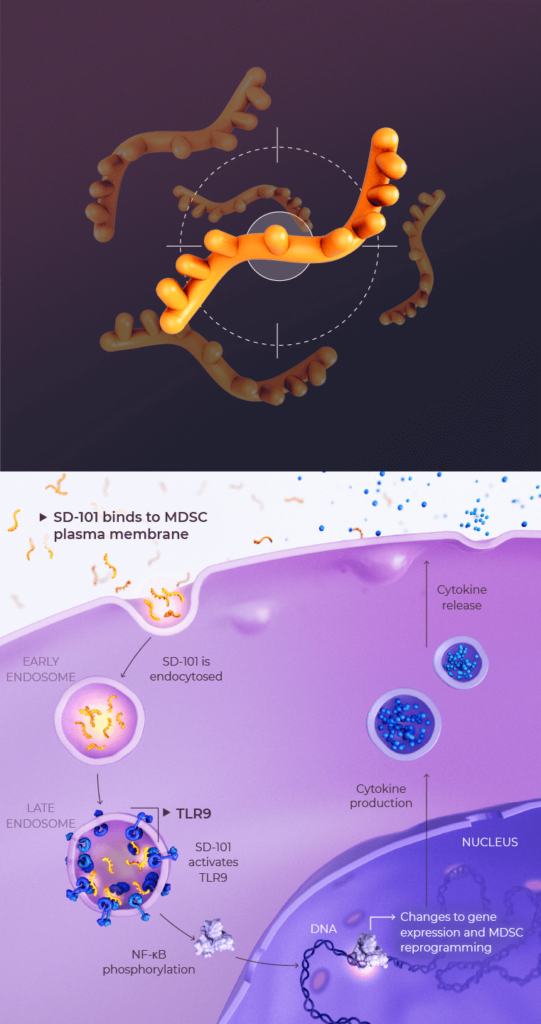
Nelitolimod, an investigational agent in development, is a toll-like receptor 9 (TLR9) agonist which is believed to bind to the TLR9 receptors found on suppressive immune cells including myeloid-derived suppressor cells (MDSCs), antigen-presenting immune cells and other immune cells. Toll-like receptors play a key role in the innate immune system and create a bridge to adaptive immunity.1 It is believed that activating TLR9 primes immune cells to promote anti-tumor T-cell function.1,2
Nelitolimod has been studied in combination with pembrolizumab (Keytruda®) and other agents in over 300 patients enrolled in various Phase 1 and Phase 2 studies of melanoma, lymphoma, and head and neck squamous cell carcinoma.3,4 It was also studied in the I-SPY2 Trial for patients with high-risk, Stage II/III breast cancer.
Traditionally, TLR9 agonists like nelitolimod have not been administered intravenously but by direct injection into superficial tumors, making treatment of tumors in deep locations, like the liver and pancreas, difficult. TriSalus will utilize its proprietary Pressure-Enabled Drug Delivery™ (PEDD™) method for intravascular regional delivery of nelitolimod to reach these locations.
By infusing nelitolimod via our technology, we can administer it to visceral organs and directly to the site of disease. The goal of this approach is to enhance nelitolimod’s therapeutic index, increase anti-tumor immune activity intended to slow tumor progression and restore, enable or improve responses to immunotherapies for treatment of liver metastases and pancreatic cancer.
Our intravascular regional delivery technology overcomes physical barriers to delivery.
TriSalus will initially evaluate nelitolimod for the treatment of uveal melanoma with liver metastases, hepatocellular carcinoma and intrahepatic cholangiocarcinoma. We intend to develop additional programs looking at a combination of nelitolimod and other immuno-oncology agents delivered with Pressure-Enabled Drug Delivery™ (PEDD™) in a range of liver and pancreatic cancers for which limited therapeutic options exist.
We are committed to developing treatments to improve outcomes for patients with liver and pancreatic tumors.
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.