Pressure inside the tumor causes surrounding blood vessels to collapse, preventing therapy from reaching higher-pressure regions within the tumor.6 When drugs are delivered suboptimally, the therapeutic index may be insufficient to achieve the desired effect or higher doses may be needed to accomplish a therapeutic effect. This may lead to potential adverse events.
Pressure inside the tumor causes surrounding blood vessels to collapse, preventing therapy from reaching higher-pressure regions within the tumor.6 When drugs are delivered suboptimally, the therapeutic index may be insufficient to achieve the desired effect, or higher doses may be needed to accomplish a therapeutic effect, leading to potential adverse events.
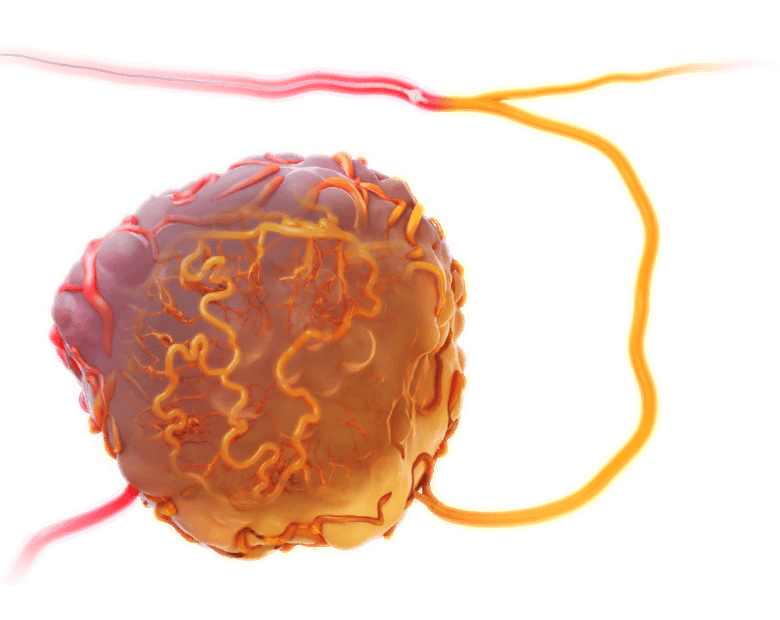
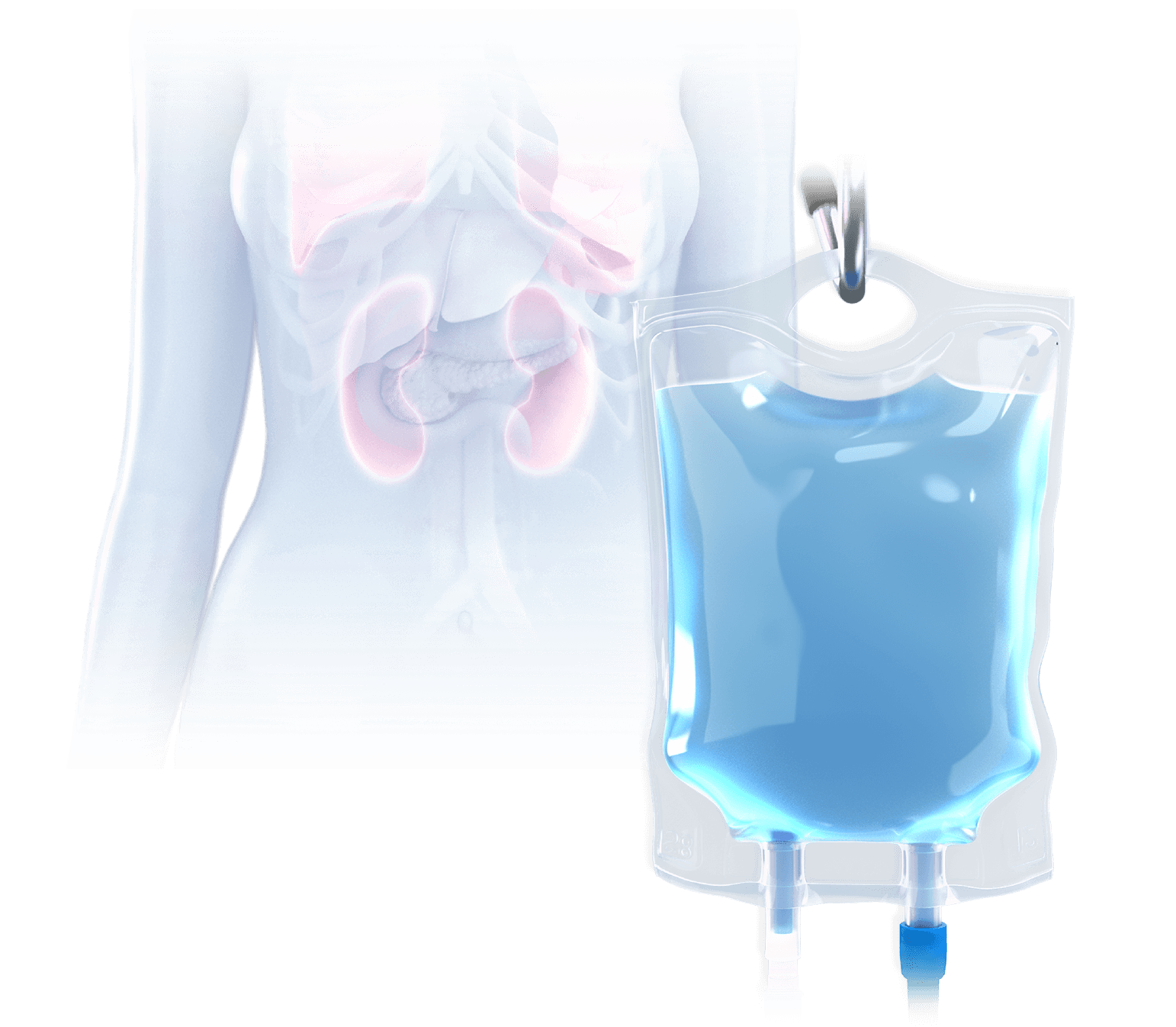
With systemic IV infusion, it is difficult to achieve therapeutic levels within the tumor and significant concentrations of drug frequently accumulate in healthy tissue, leading to severe side effects and dose-limiting toxicity.7
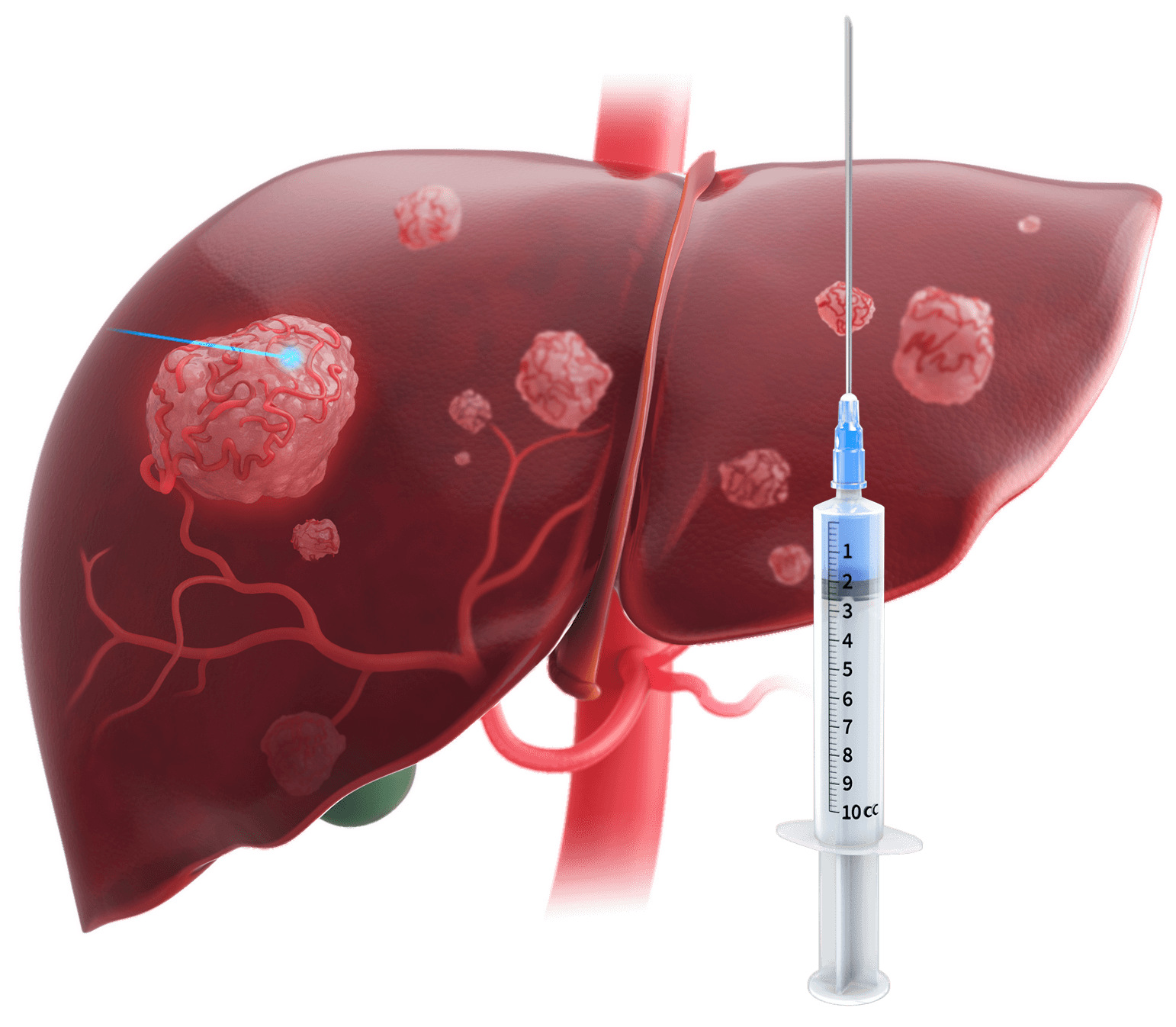
With local needle injection of a drug, therapy is highly localized at the delivery site and not distributed throughout the tissue. Non-injected adjacent tissues or lesions receive little to no therapy.8

Intratumoral saturation of therapy may not be achieved with microcatheters as pressure and flow cannot be modulated to overcome ITP, limiting therapeutic uptake.2
The use of a balloon catheter stops forward blood flow and reduces pressure to the distal vasculature, impacting therapy distribution and uptake in target tissues.9,10
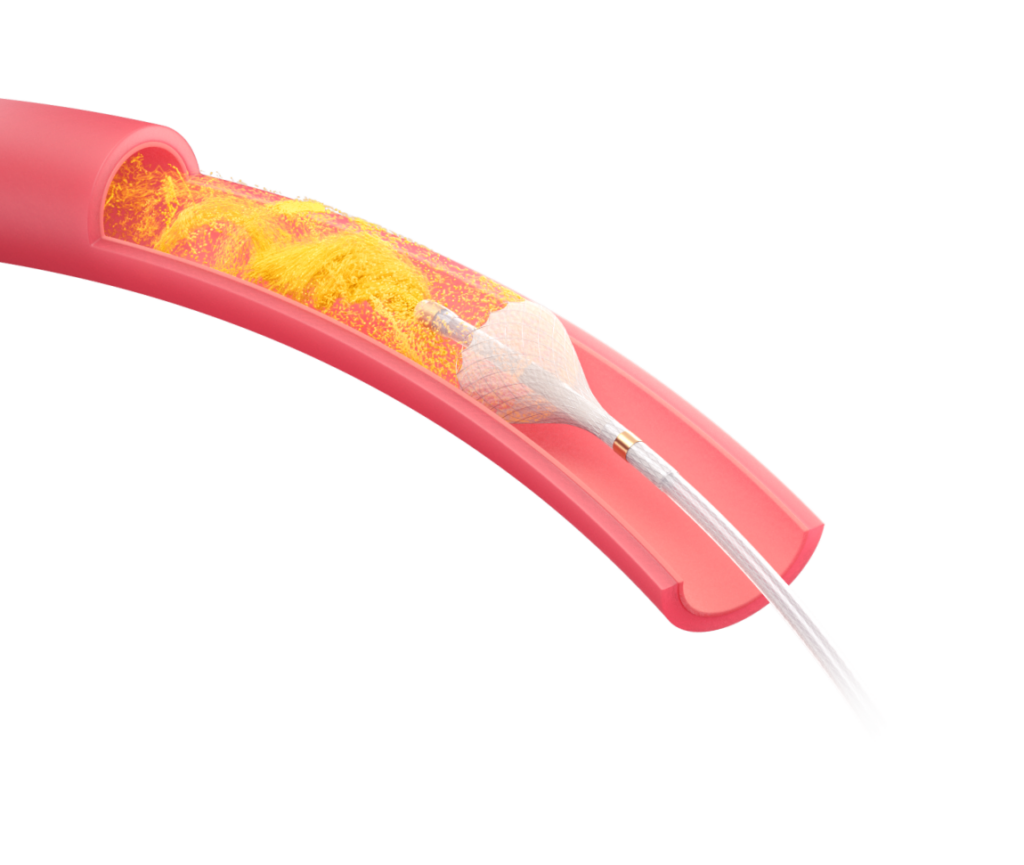
TriSalus’ platform addresses two significant barriers to therapy efficacy in the liver and pancreas. Pressure-enabled delivery of immune-enabling therapies may provide a means to treat these hard-to-reach tumors and improve treatment outcomes.
A retrospective analysis of 61 patients with liver cancer (190 lesions) treated with resin Y90 RE showed PEDD significantly improved both tumor targeting and dose delivery.
In this study, each patient served as their own control. All patients underwent a MAA planning procedure delivered via a standard end-hole (EH) microcatheter. Resin Y90 was then delivered via either an EH microcatheter (control group) or via PEDD, followed by PET/CT imaging. Each patient’s post-Y90 PET/CT was co-registered to their post-MAA SPECT/CT to compare the tumor to normal liver ratio (T/N) and tumor dose (TD).
Overall, 38 patients (115 lesions) and 23 patients (75 lesions) were analyzed in the PEDD and control groups, respectively. The results showed that across all tumor types, PEDD increased the T/N by a median of 24%, and the TD by a median of 23%, with no significant difference seen in the standard EH (control) group.
Read about the innovative research conducted by our scientists and clinical collaborators.
In this small, retrospective study of patients with solitary hepatocellular carcinoma (HCC), drug-eluting microspheres transarterial chemoembolization (DEM-TACE) procedures with PEDD were associated with improved tumor response.
Patients who received PEDD showed better objective response (100% vs. 76.5% with standard EH, P < 0.05) and pathological response (88.8% ± 2.5% tumor necrosis after 1 treatment with PEDD, vs. 33.8% ± 41.1% with EH, P < 0.05). Pathological analysis of explanted livers showed greater concentrations of microspheres within the tumor relative to the surrounding tissue in PEDD explants (88.7 ± 10.6%) versus the EH explants (55.3 ± 32.7%) (p = 0.002).
End-hole Versus Microvalve Infusion Catheters in Patients Undergoing Drug-Eluting Microspheres-TACE for Solitary Hepatocellular Carcinoma Tumors: A Retrospective Analysis
This small, prospective study evaluated differences in the hepatic distribution of embolic particles following infusion with a standard EH microcatheter versus the PEDD method. The procedure was performed on each patient on the same day, with the same tumor, and in the same catheter location.
Decreases in hepatic non-target embolization were found in all patients when PEDD was used, representing a 24%–89% reduction (P <0.05). Increased tumor deposition was also noted in all patients, representing a relative increase of 33%–90% (P <0.05).
This small study demonstrated that hepatic artery infusion of an investigational CAR-T using the PEDD method resulted in increased delivery of CAR-T to the tumor and showed encouraging activity against CEA+ liver metastases.
Compared to previous CAR-T HAI trials with an EH microcatheter, PEDD significantly increased the frequency of CAR-T by 5.2-fold within LM, as detected by quantitative PCR (p=0.03). All patients showed a decrease in CEA post-treatment with a mean CEA decrease of 15ng/ml (3-39 ng/ml). Two out of five patients demonstrated complete PET response in the liver.
aD’Abadie et al.:
A retrospective analysis of 61 patients who underwent resin Y90 RE for primary or metastatic liver tumors. Each patient served as their own control. All patients underwent a 99mTc-MAA planning infusion delivered via EH microcatheter, followed by SPECT/CT imaging. One to three weeks after the planning angiography, resin Y90 microspheres were delivered via PEDD or via a standard EH microcatheter (control group) at the discretion of the treating interventional radiologist. PET/CT imaging was performed immediately following RE.11
bTitano et al.:
A retrospective, single-center study included 88 treatment-naive patients with solitary HCC tumors <6.5 cm who underwent treatment utilizing either the Surefire® Infusion System (SIS) with SmartValve® technology (n = 18) or standard EH microcatheters (n = 70). Twenty-three patients (5 SIS, 18 EH) received a liver transplant during the study, with 1 SIS and 6 EH patients excluded from the tumor necrosis analysis for receiving subsequent therapies prior to transplant.2
cPasciak et al.:
A prospective study included 9 patients with unresectable liver cancer who were enrolled for the treatment of HCC (n = 6), liver-dominant metastatic disease (n = 2) or intrahepatic cholangiocarcinoma (n = 1). Each patent was treated with Tc-99m MAA (macroaggregated albumin) via standard EH microcatheter or PEDD.3
dKatz et al.:
An exploratory Phase 1b clinical trial using biopsy to assess CAR-T activity of a second-generation (IgCD28TCR) anti-CEA CAR-T (Sorrento Therapeutics) administered using HAI via PEDD in five patients with stage IV, chemotherapy-resistant, CEA+ adenocarcinoma liver metastases (LM). Patients received 3 HAI of 10^10 anti-CEA CAR cells and low dose IL-2 (50,000 IU/kg/day) (NCT02850536).11
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.