
TriSalus takes an integrated approach tailored to the target organ and disease biology, with the goal to overcome two critical barriers: immunosuppression and delivery.
Watch to learn how our platform approach applies to the treatment of liver tumors.
0:39 min
Share video

At TriSalus, we address two significant barriers to efficacy of immunotherapy in the liver, immunosuppression and delivery, by delivering immune-enabling therapy with the Pressure-Enabled Drug Delivery™ (PEDD™) method.
Watch to learn how our platform approach applies to the treatment of liver tumors.
1:59 min
Share video

Our investigational therapeutic candidate nelitolimod has the potential to reprogram or eliminate immunosuppressive cells and enable immunotherapy in liver tumors.
Watch to learn how our platform approach applies to the treatment of liver tumors.
1:27 min
Share video

The tumor microenvironment (TME) diminishes the efficacy of immunotherapies through high intratumoral pressure (ITP) and vascular collapse. Our Pressure-Enabled Drug Delivery™ (PEDD™) method is designed to overcome ITP and improve therapeutic delivery in liver tumors.
Watch to learn how our platform approach applies to the treatment of liver tumors.
1:17 min
Share video

TriSalus’ Pressure-Enabled Drug Delivery™ (PEDD™) method has the potential to overcome some of the challenges seen in traditional drug delivery approaches.
Watch to learn how our platform approach applies to the treatment of liver tumors.
1:34 min
Share video

TriSalus’ Pressure-Enabled Drug Delivery™ (PEDD™) method has the potential to overcome some of the challenges seen in traditional drug delivery approaches.
Watch to learn how our platform approach applies to the treatment of liver tumors.
1:34 min
Share video
The TriSalus™ platform has the potential to overcome the barriers that limit treatment success for liver and pancreatic tumors. Through our work, we aim to enable more patients to benefit from checkpoint inhibitors and other immunotherapeutic agents. To learn more about our current work, see our pipeline page.
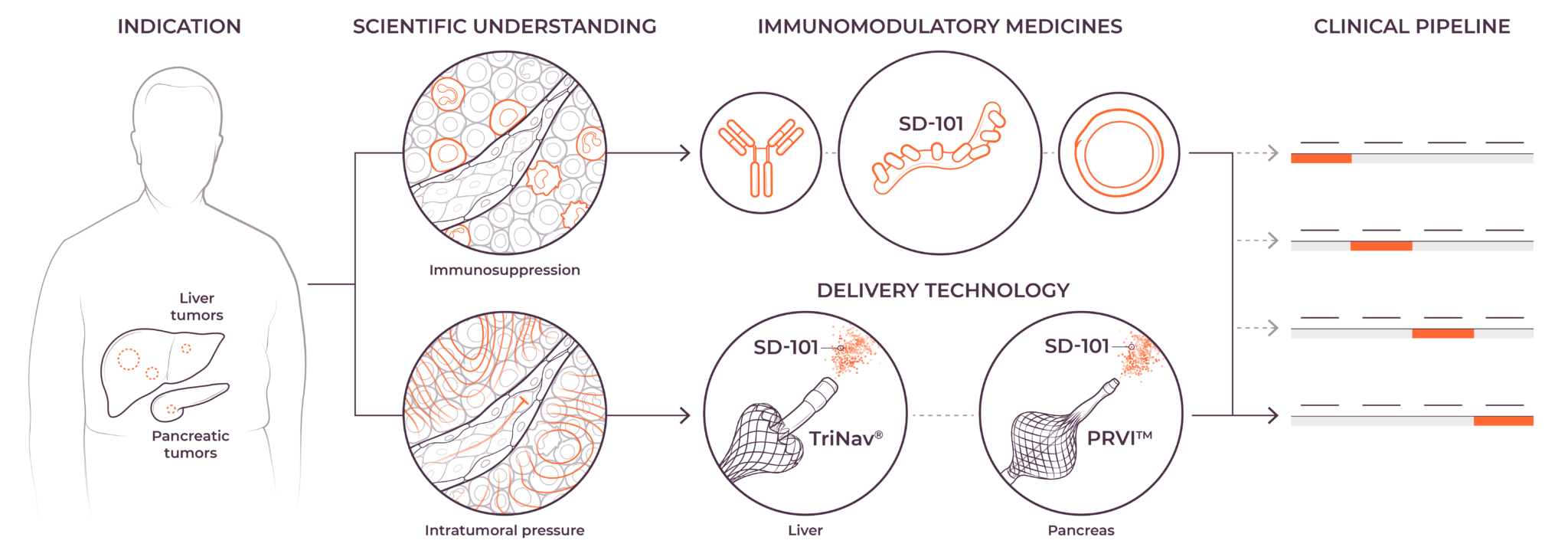
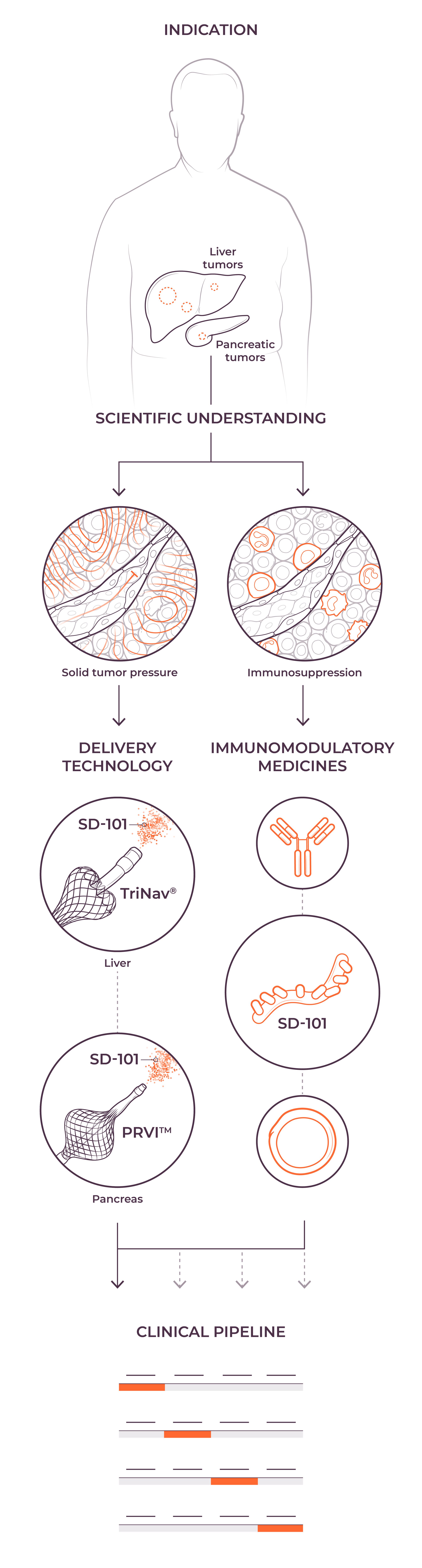
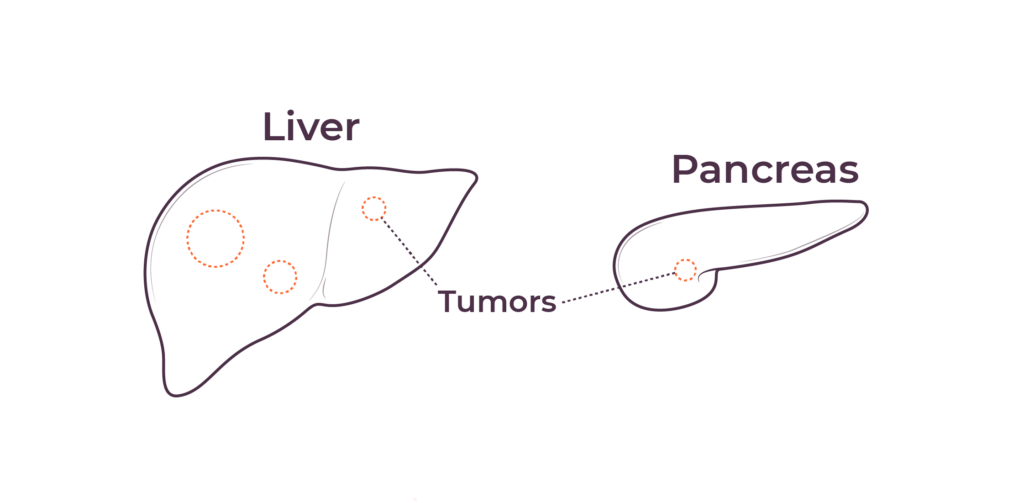
TriSalus is focused on indications that are characterized by immune dysfunction at an organ and tumor level, including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), pancreatic ductal adenocarcinoma (PDAC), PDAC with liver metastases, uveal melanoma with liver metastases, and colorectal cancer with liver metastases.
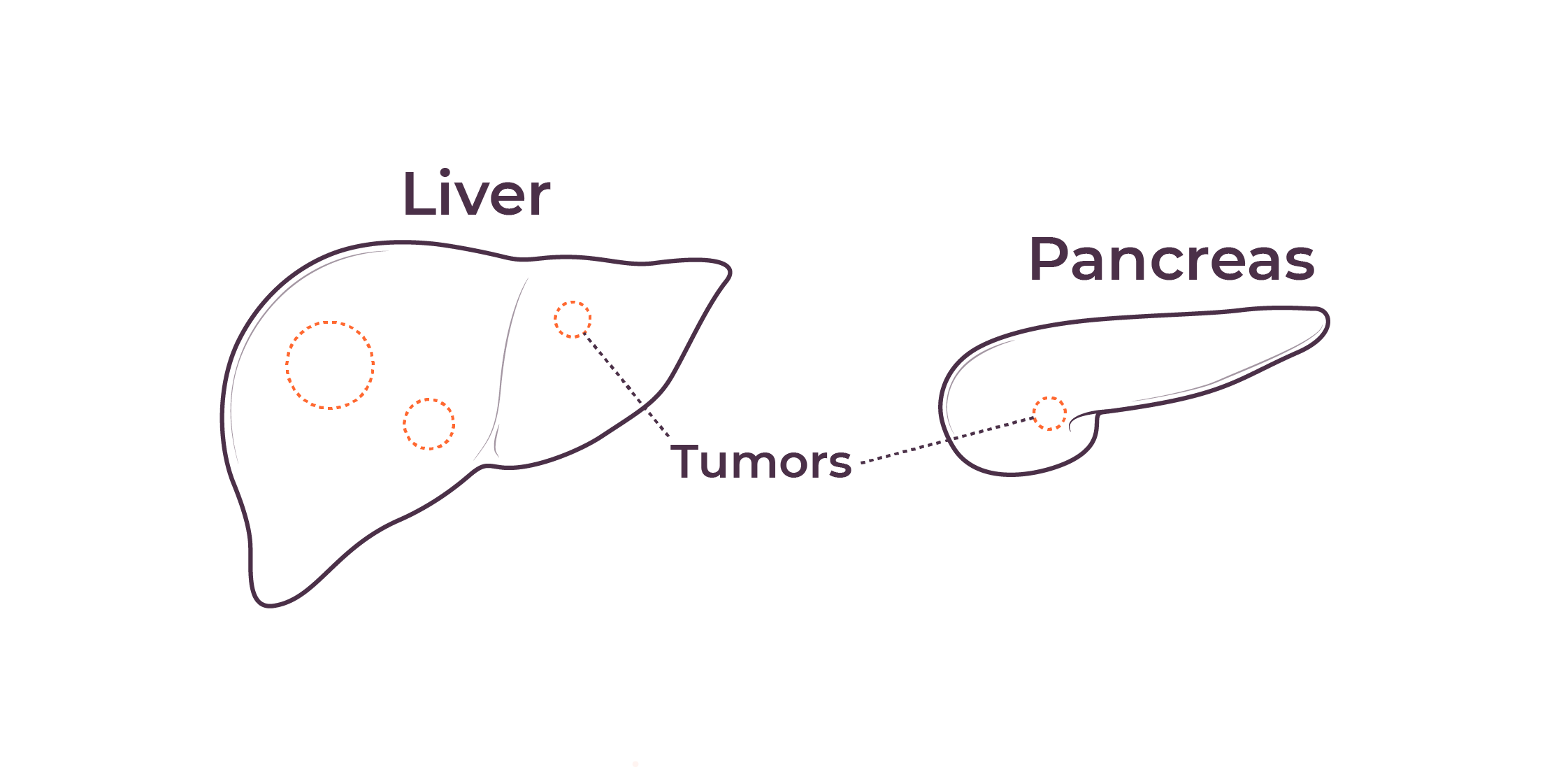
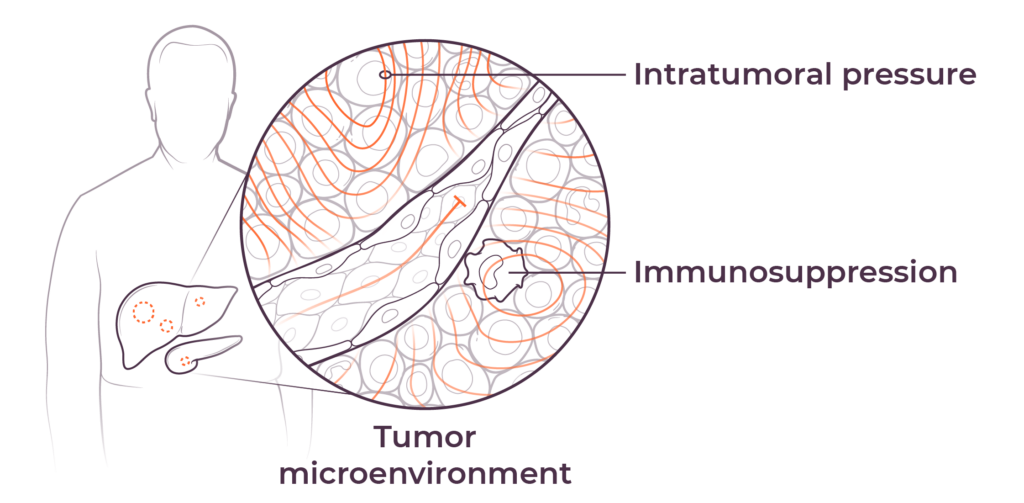
We believe that poor outcomes in liver and pancreatic cancers are due to immune dysfunction specific to the liver and the tumor microenvironments in the liver and pancreas, as well as high intratumoral pressure which limits delivery efficacy. These barriers play a key role in preventing consistent and durable treatment responses.
At TriSalus, we are dedicated to researching these organ-specific issues to develop better treatments.
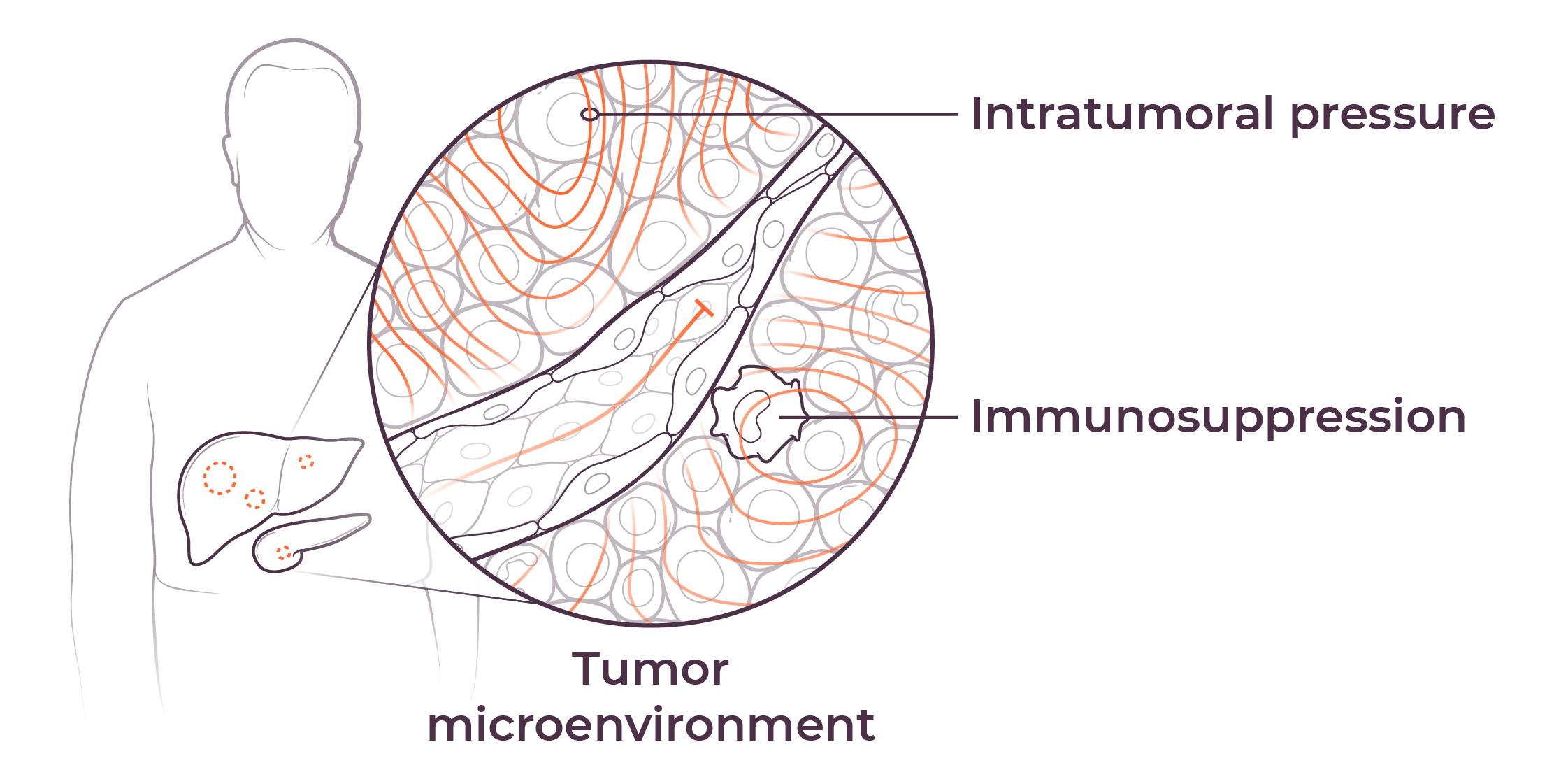

Our investigational agent nelitolimod, a selective toll-like receptor agonist, has demonstrated potential to reverse immunosuppression and promotion of responses to other forms of immunotherapy in early studies.1,2 We are presently developing nelitolimod to enable immunotherapy for patients with liver metastases, primary liver tumors and pancreatic cancer. In addition, we intend to in-license or acquire other immuno-oncology products and other tumor-killing agents to combine with nelitolimod to further improve patient outcomes.

We are combining nelitolimod with our PEDD method to improve intravascular therapeutic delivery by modulating pressure and flow to enhance local drug concentrations in tumors. In preclinical and clinical studies, PEDD has demonstrated increased therapeutic delivery and improved tumor response in the liver across a range of modalities and geographies.3–8
We are working tirelessly to conduct clinical trials to develop new treatments for patients. Our drive for innovation is fueled by our passion for patients.
1. Looi CK, et al. J Exp Clin Cancer Res. 2019;38(1):162.
2. Ribas A, et al. Cancer Discov. 2018;8(10):1250-1257.
3. Titano JJ, et al. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
4. Pasciak AS, et al. J Vasc Interv Radiol. 2015;26(5):660-669.
5. Katz SC, et al. J Immunother Cancer. 2020;8(2):e001097.
6. d’Abadie P, et al. Diagn Interv Radiol. 2021;27(6):768-773.
7. Data on File, Porcine Animal Model, TriSalus Life Sciences®, 2019.
8. Shankara Narayanan JS, et al. Surgery. 2020;168(3):448-456.
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.