We currently have two FDA-cleared devices. To learn more about our platform combining pressure-enabled delivery with immunomodulatory therapeutics, visit our platform page.
Our technology has been shown to overcome intratumoral pressure (ITP) and enable targeted drug delivery of therapeutics to primary liver tumors and liver metastases via the peripheral vascular system. The technology enables therapeutic delivery to all tumors in the liver in addition to micro-metastases and dysfunctional immune cells throughout the organ.
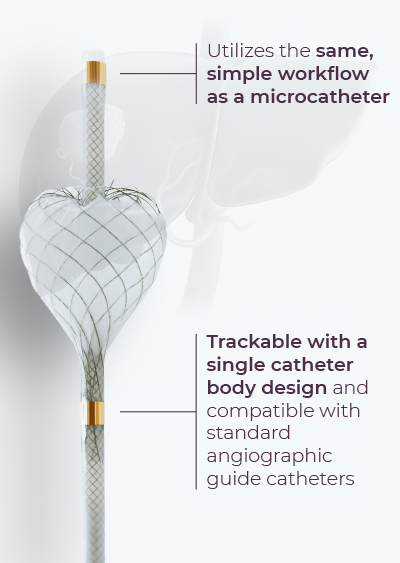
The FDA-cleared TriNav infusion system with SmartValve® technology enables delivery of therapeutic agents to selected sites in the peripheral vascular system, including tumors in the liver and the entire organ in the presence of micro-metastases.
Retrograde venous delivery to the pancreas involves the placement of a PEDD device into the veins draining the pancreas to enable targeted delivery of therapeutics. Unlike the liver, small vessels and extensive collateralization in the pancreas make the arterial route challenging for targeted delivery. Retrograde venous infusion provides a potentially more feasible and reliable strategy for targeted delivery of therapeutics through direct venous access.

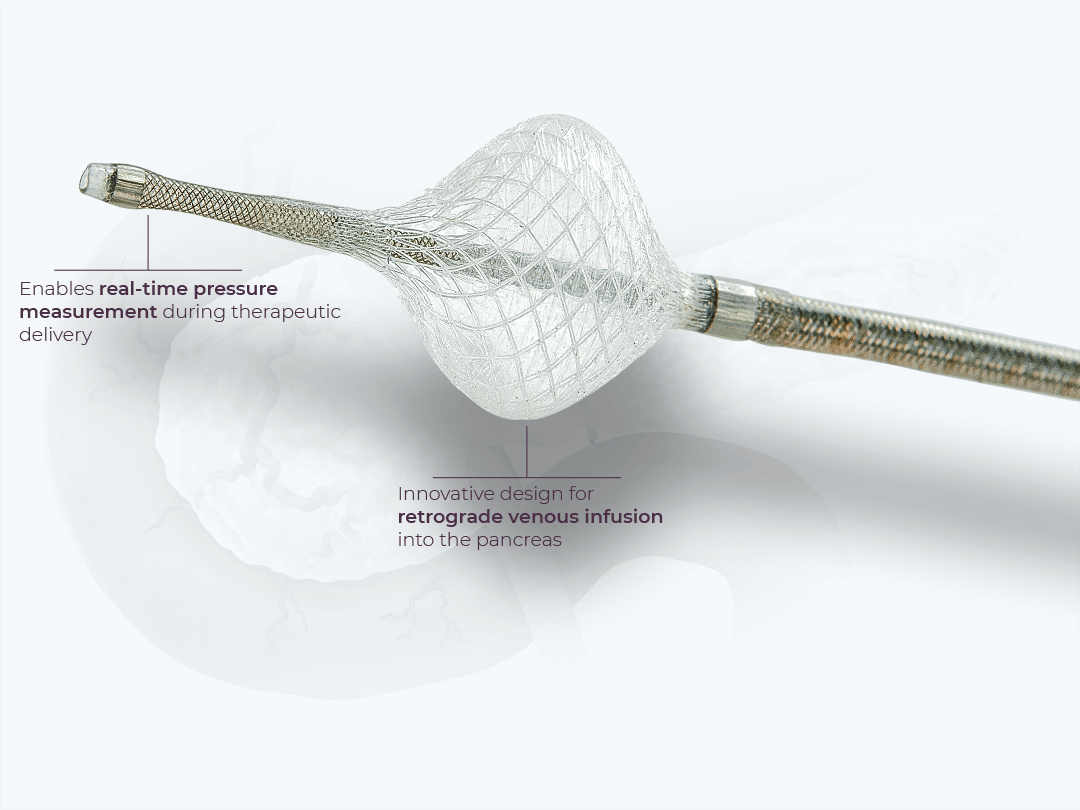
The PRVI System with SmartValve technology is an FDA-cleared device for delivery of therapeutics to the peripheral vasculature. This device is being studied for the delivery of SD-101 via the PRVI approach into unresectable pancreatic tumors.
The PRVI System is positioned in the vasculature using standard interventional radiology procedures. The PRVI System isolates the tumor bed and enables pressure measurement during infusion for uniformity of procedural approach.

The PRVI System with SmartValve technology is an FDA-cleared device for delivery of therapeutics to the peripheral vasculature. This device is being studied for the delivery of SD-101 via the PRVI approach into unresectable pancreatic tumors.
The PRVI System is positioned in the vasculature using standard interventional radiology procedures. The PRVI System isolates the tumor bed and enables pressure measurement during infusion for uniformity of procedural approach.
At TriSalus, we believe that a therapeutic approach that simultaneously reduces immunosuppressive populations and improves therapeutic delivery to tumor tissue holds great promise for patients with few good options.
We encourage you to read and evaluate terms of use, privacy, security and other similar policies of the destination site as they may differ from TriSalus’ standards.
TriSalus assumes no responsibility nor does it control, endorse or guarantee any aspect of your use of any third party sites. Additionally, the presence of this link does not imply the third party site’s endorsement of TriSalus or this website.
Thank you for visiting our site.